DSCSA
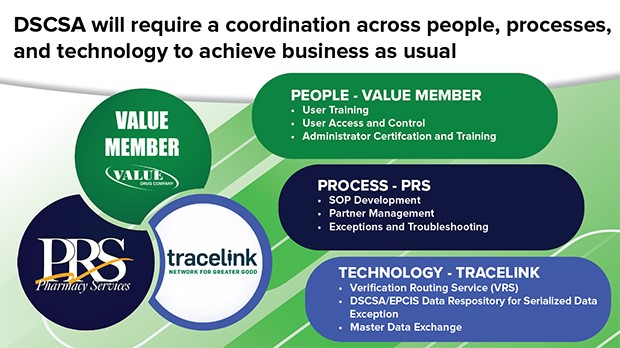
Value Drug Partners with TraceLink and PRS to Provide Full-Service DSCSA Solution Value Drug Company has established a partnership with TraceLink and PRS to provide Value Drug members with a comprehensive DSCSA solution that aims to meet the requirements that will go into effect on November 27, 2023. These partners will offer services to help you create the required written standard operating procedures (SOPs) to be compliant and offer the technical solution/portal(s) to accommodate the required product tracing, verification, and exchange of the EPCIS transaction data. Over the course of several months, Value Drug researched many service providers and determined that PRS and TraceLink were best equipped to meet our members' needs and help you remain compliant with DSCSA.
If you’re a Value Drug member and you would like to learn more about the DSCSA partnership, please visit ValueDrugHub > Library > DSCSA.
Value Drug Partners with TraceLink and PRS

